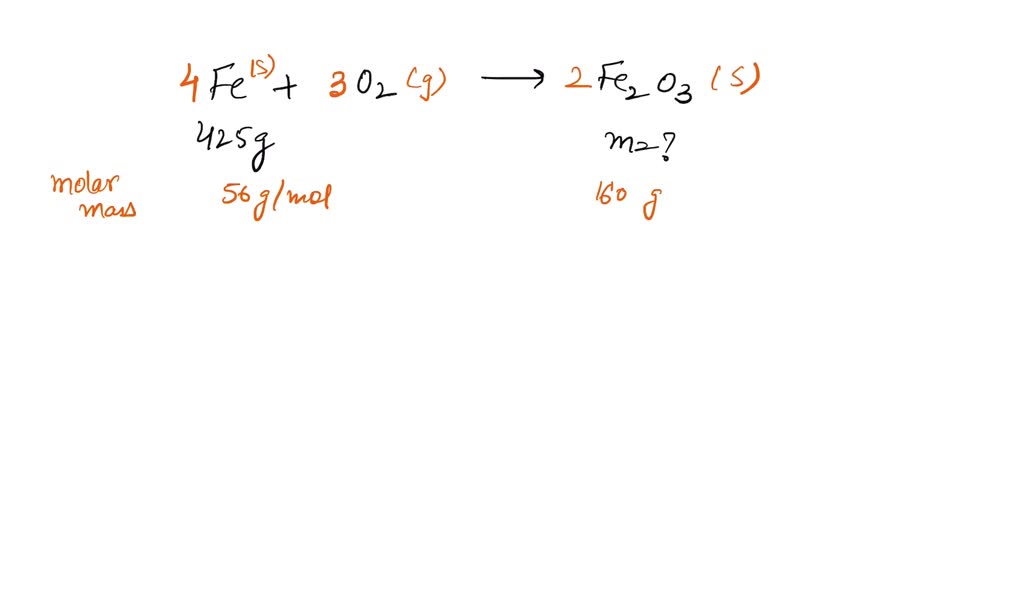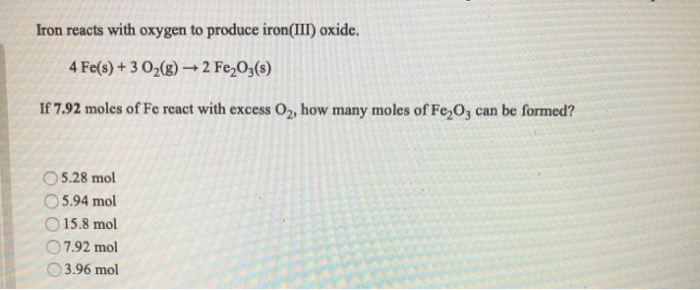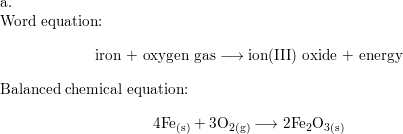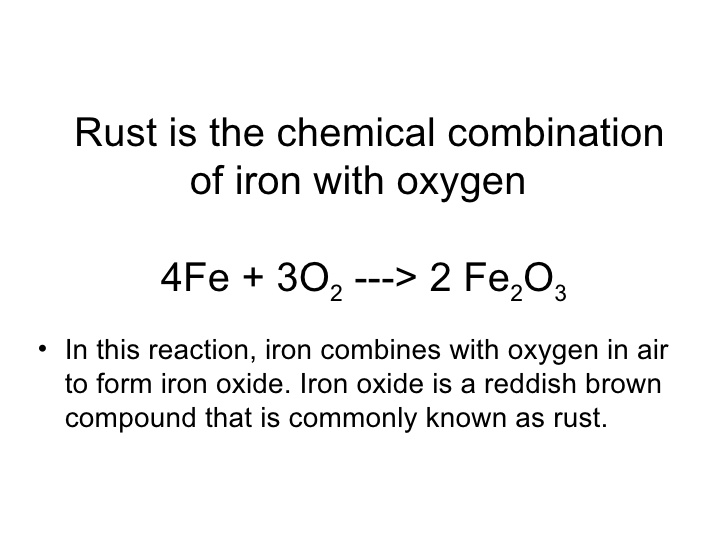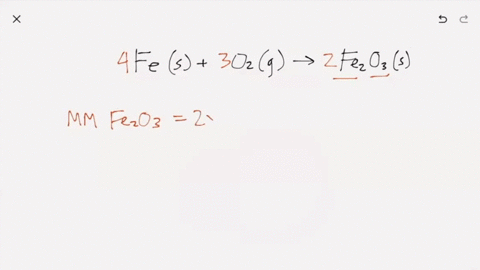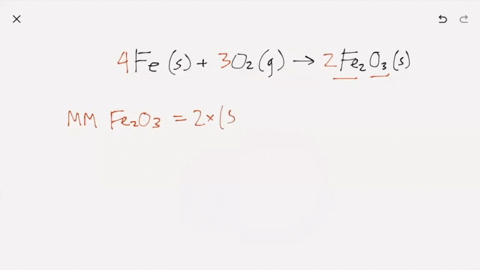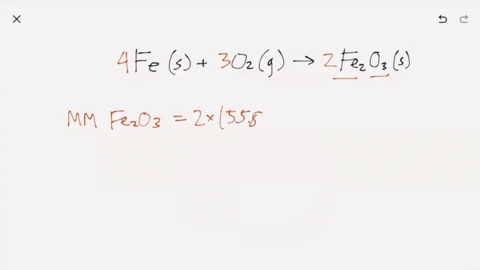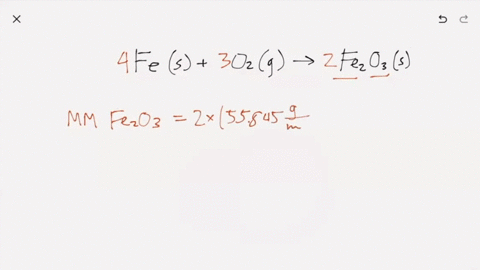
SOLVED:Iron metal reacts with oxygen to give iron(III) oxide, Fe2 O3 (a) Write a balanced equation for the reaction. (b) If an ordinary iron nail (assumed to be pure iron) has a

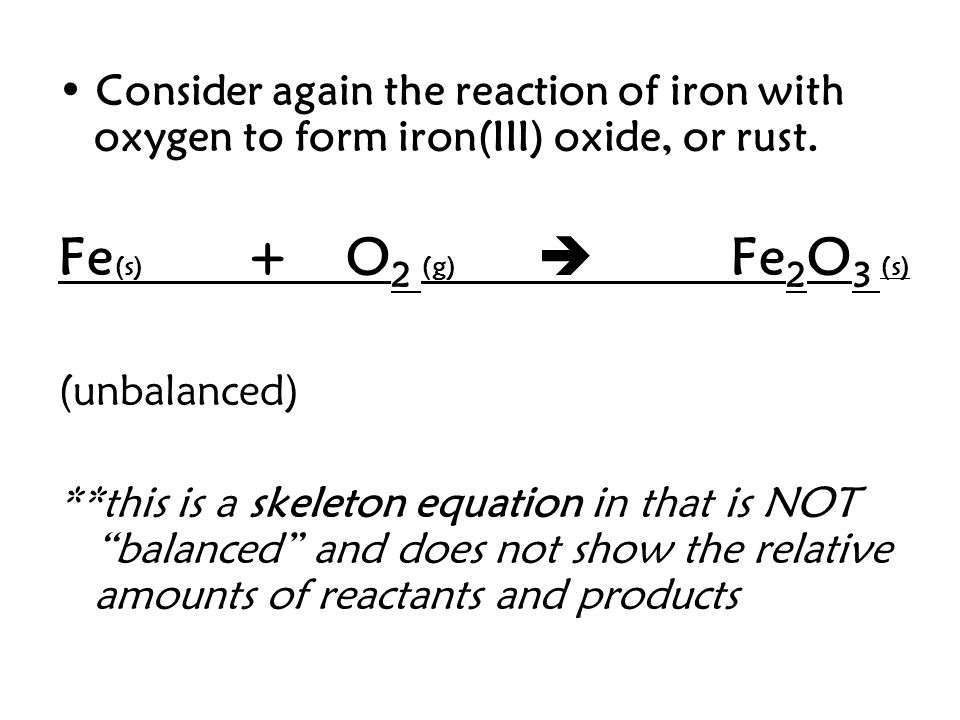
What does it mean for an equation to be balanced? We have the same number of each type of atom on each side of the equation. Make sure you have balanced. -

⚗️Iron (Fe) reacts with oxygen gas (O2) to form rust (Fe2O3). Balance the equation below by writing - Brainly.com

What is oxidation of iron in inorganic chemistry? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium